
ISO 15189 Certification
Google User
Rating: 5 out of 5
ISO 15189 Certification
ISO 15189 Certification is a comprehensive standard specifically meant for MEDICAL LABORATORIES. It covers the Management, staff, customers, physical lab, error prevention and management towards achieving laboratory quality. Expert Certifier performs compliance verification and certifies the medical laboratory towards ISO 15189 Certification standards as per the Expert Certifier standard audit procedure.
ISO 15189 certification is a laboratory accreditation standard. IAS coordinates with UCAS to deliver the laboratory accreditation. It is a comprehensive standard specifically meant for MEDICAL LABORATORIES.

ISO 15189 Certification Principles
- Laboratory Management has a responsibility to manage
- The laboratory must know who are its users (customers) and meet their requirement
- The physical laboratory should not interfere with laboratory workers or laboratory samples
The requirements of this standard cover the following aspects:
Management, staff, customers, physical lab, error prevention and management towards achieving laboratory quality.
MANAGEMENT REQUIREMENTS
- Organization and management responsibility
- Quality management system
- Document control Service agreements
- Examination by referral laboratories
- External services and supplies
- Advisory services
- Resolution of complaints
- Identification and control of nonconformities
- Corrective action Preventive action
- Continual improvement Control of records Evaluation and Audits Management review.
TECHNICAL REQUIREMENTS
- Personnel
- Accommodation and environmental conditions
- Laboratory equipment,
- reagents, and consumables
- Pre-examination processes
- Examination processes
- Ensuring the quality of examination results
- Post-examination processes
- Reporting of results Release of results
What does ISO 15189 Certification mean?
ISO 15189 Certification Medical laboratories — Requirements for quality and competence is an international standard that specifies the quality management system requirements particular to medical laboratories. The standard was developed by the International Organisation for Standardization’s Technical Committee 212 (ISO/TC 212).
ISO CONSULTANT | OUR MISSION
Understanding the customer requirement and meeting it as per the agreed terms. Deliver consultancy with transparency and simplicity. Diversify into new areas that compliment and supplement the core business, with the diversification aimed at achieving excellence and industry leader status in the new areas.
Matrix will be the most favourite consulting firm by the customers for all their management support. It will be valued and respected for its professional, innovative, profitable information, and knowledge based approach. With the objective of growing into a regional and global player, Matrix shall demonstrate a clear concern for ethical conduct with customer, employee and shareholder interests at the core of its operations
ISO 15189 Certification is the International standard, specifies requirements for quality and competence in medical laboratories. ISO 15189 can be used by medical laboratories in developing their quality management systems and assessing their own competence. It can also be used for confirming or recognising the competence of medical laboratories by laboratory customers, regulating authorities and accreditation bodies.
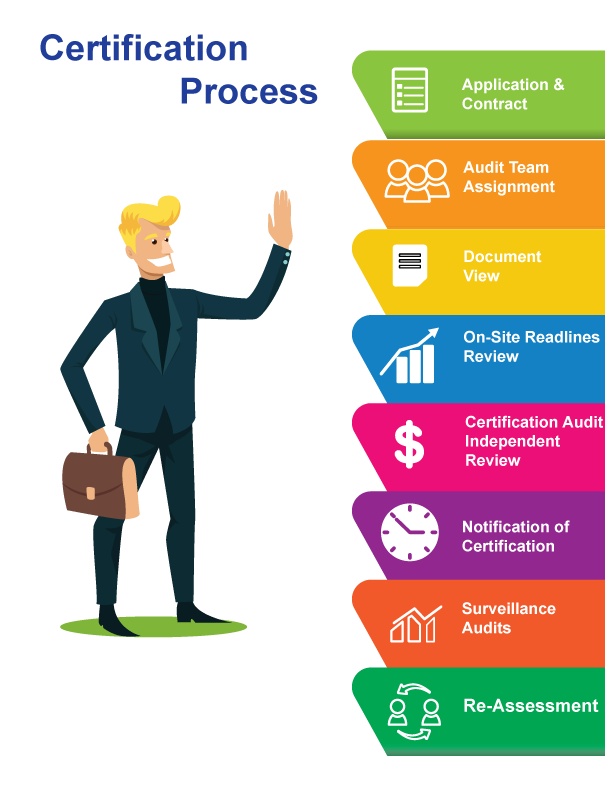
Our advice, go for it!!!
If you are looking or thinking on how to get ISO 15189 certification in India you can reach out to us. You can partner with us on consulting the standard requirement. We help our customers to consult free of cost. You can reach out to us at or write to us on contact@expertcertifier.com with your entire certification requirement. Also, you can feel free to visit our official website at www.expertcertifier.com and provide your contact details so that one of our Consulting experts can contact you in order to understand more about your process so that we can perform a free gap analysis. We are available 24/7 for all of our customers.





















